What are Telomeres?
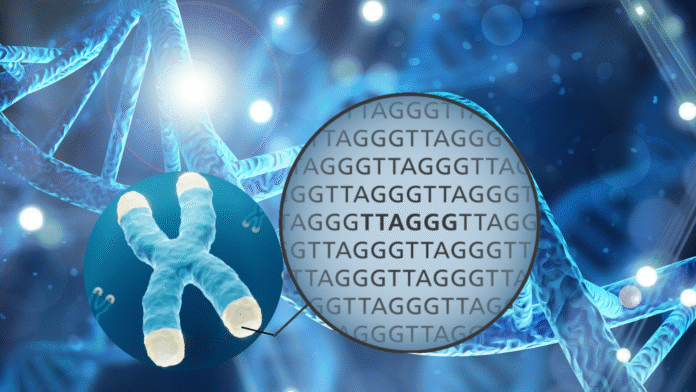
The sequence of bases in the coding region of DNA makes up the genes, which provide information or instructions for building various proteins, which are essential for the body’s functions and also determine our traits and characteristics. Besides the coding region, DNA also comprises non-coding base sequences called “telomeres.” Telomeres are the protective caps of short repetitive DNA sequences at the ends of chromosomes that do not contain active genes and are not transcribed into proteins. Instead, they prevent DNA damage by preventing chromosome ends from sticking together, entangling or unwanted fusion with other chromosomes.
Shortening of Telomere Length
The average length of telomere in healthy human cells is around 5 kilobases at the age of 60–70 years while 20 kilobases at the age of <10 years. With each cell division, telomeres loses about 50-200 base pairs because the end of DNA is not fully replicated and some base repeat sequences are cut off. That’s why with successive cell division, the telomeres become shorter and shorter and eventually, they become so short that the cell can no longer divide and dies. Length of telomeres is not reduced in cells which do not divide continually such as cardiac muscle cells.
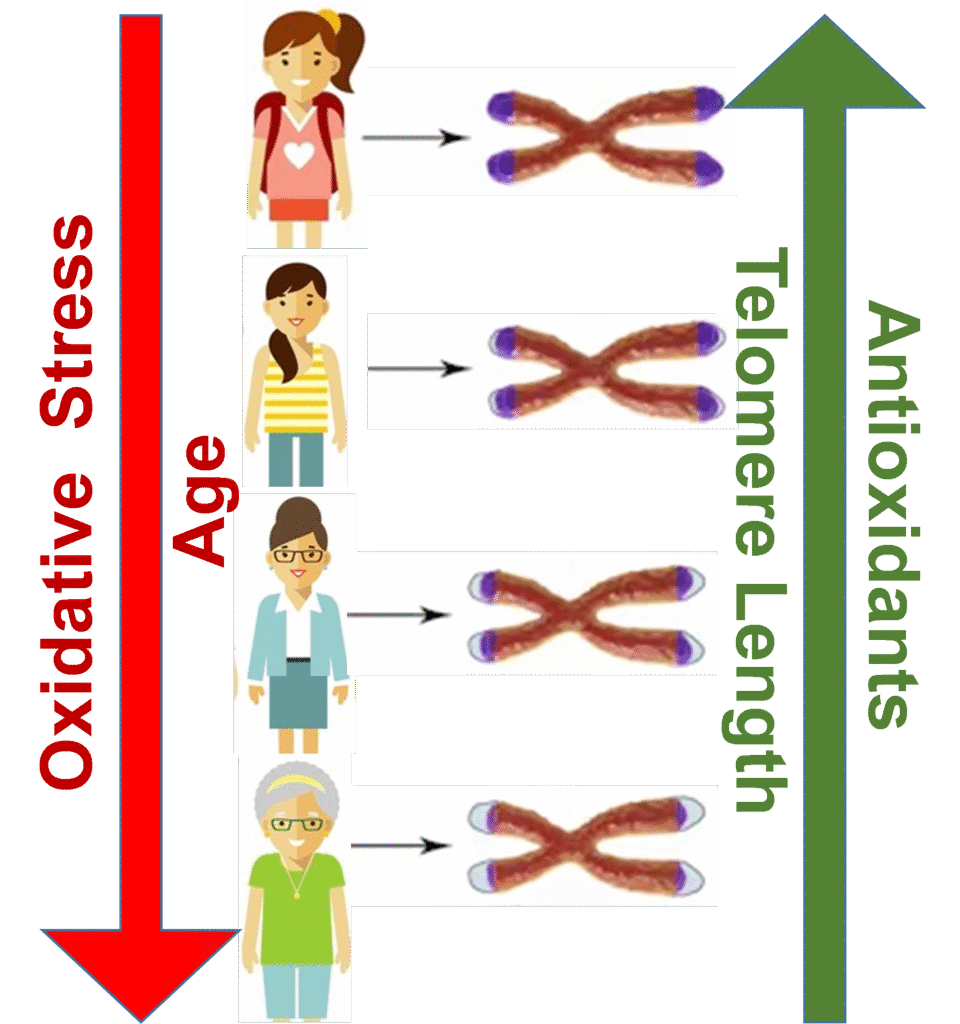
Association of Telomere Length with Longevity and Diseases
Telomere length is a biological marker for cell aging because it decreases with increased age. Research studies have shown that individuals with longer telomeres live longer than those with shorter telomeres. Decrease in telomere length with age is a normal cellular process; in humans, the rate of decrease is about 24.8–27.7 base pairs per year. However, if the pace of telomere shortening is greater than this, then a combination of multiple factors (such as smoking, obesity, lack of exercise, consumption of an unhealthy diet, oxidative stress, and deficiency of the telomerase gene) is responsible for this. Accelerated telomere shortening may result in early onset of age-associated manifestations, diseases/disorders, and life-threatening conditions, including balding, poor wound healing, intestinal disorders, bone softening, learning disabilities, Alzheimer, hypertension, cancer, diabetes, coronary heart disease and osteoporosis. The individuals with shorter leukocyte telomeres are three times more susceptible to myocardial infarction.
Obesity, Stress and Telomere Length
It is a well-known fact that obesity causes insulin resistance and increases oxidative stress. This obesity-induced oxidative stress may result in DNA damage and accelerated telomere shortening. The loss of telomeres in obese individuals has been estimated to be equivalent to 8.8 years of life. Likewise, people exposed to daily life stress have also provided evidence of oxidative damage to DNA and shorter telomeres. A difference of telomere length equivalent to 10 years of life between two groups of women, one under stress and one under normal circumstances, has been estimated.
Impact of Food/Nutrients on Telomere Length
Cross-sectional studies were performed to determine the association between various nutrients and telomere length. These studies showed positive associations between dietary fibre intake and telomere length; however, there was an inverse correlation between dietary total fat and telomere length. A retrospective case-control study showed that individuals who did not receive vitamin D treatment had shorter telomeres, while this association was not observed in other studies. In a cross-sectional study, 5.1% longer telomeres were found in women taking daily multivitamin supplements compared with the control group. Some prospective and cross-sectional studies have witnessed a positive correlation between telomere length and higher intake of fruits, vegetables, nuts, cereals, and legumes. Among beverages, tea and coffee were found to help increase telomere length, while an inverse relation between the intake of sweetened carbonated beverages and telomere length was reported. Antioxidants defend against oxidative damage; therefore, a diet rich in antioxidants such as vitamin E, vitamin C, and beta-carotene can protect telomeric DNA from damage. It has also been demonstrated in animal studies that restricted diet (eating less) leads to reduced oxidative burden and reduced DNA damage, and therefore increases animals’ lifespan. The increased lifespan is associated with longer telomeres.
Conclusion
In conclusion, chronological aging is inevitable; however, we can slow down or even reverse this natural aging process through lifestyle choices and interventions.